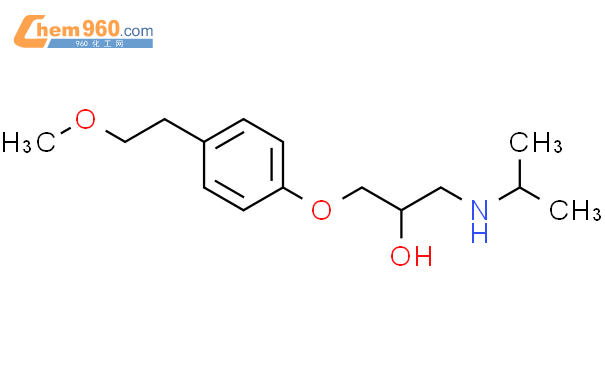
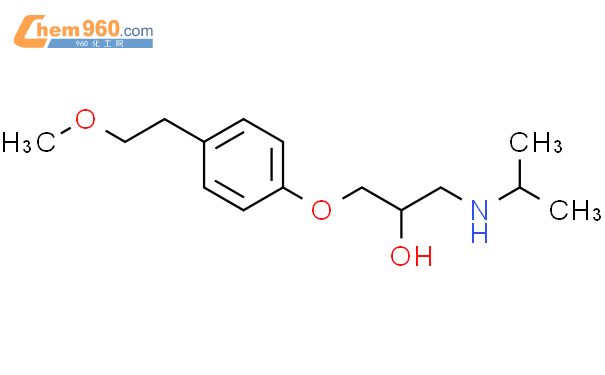
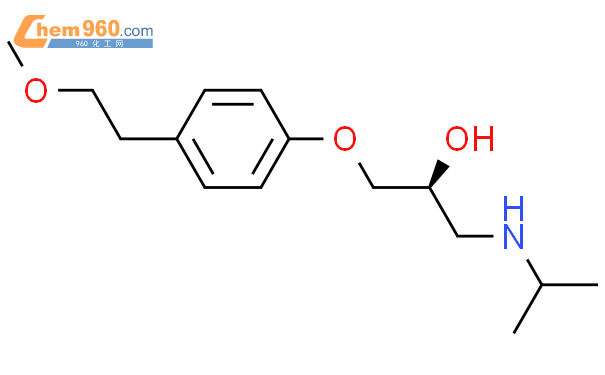
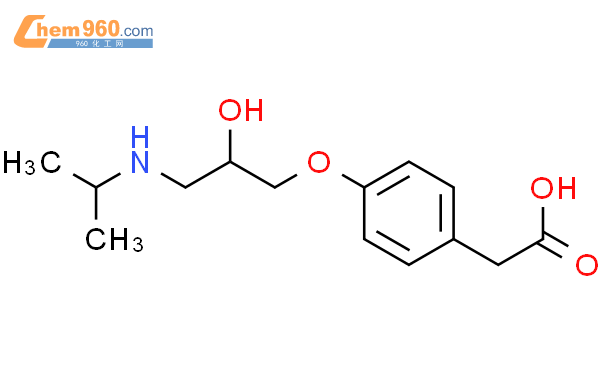
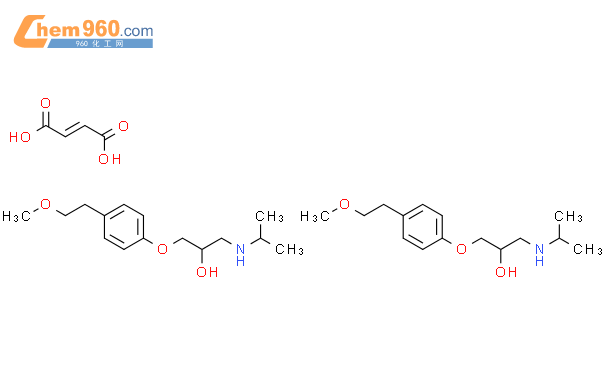
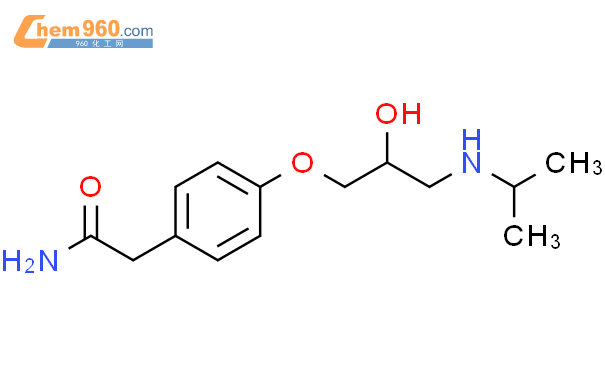
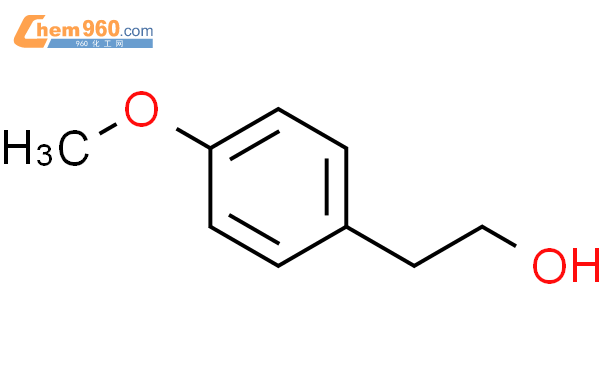
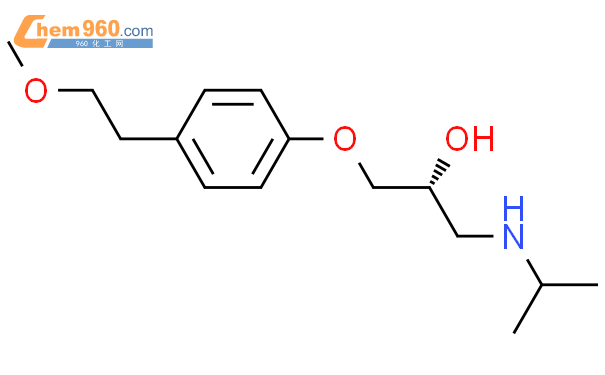
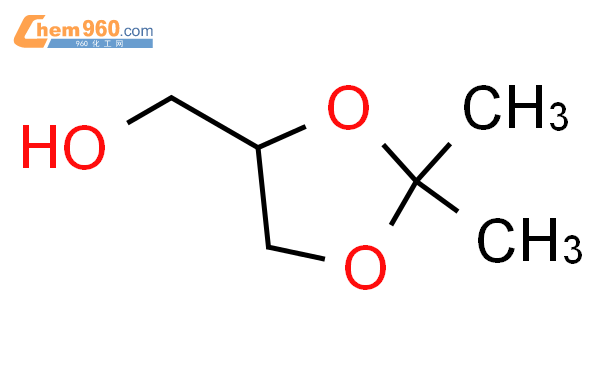
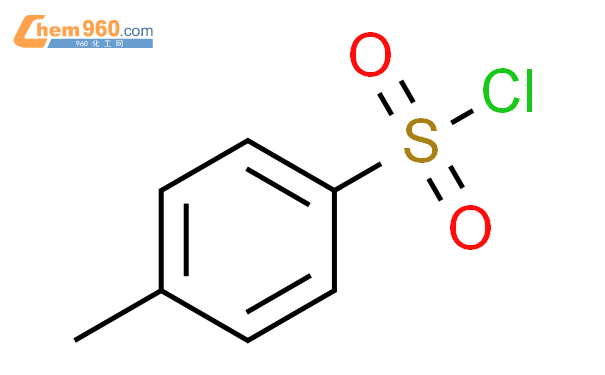
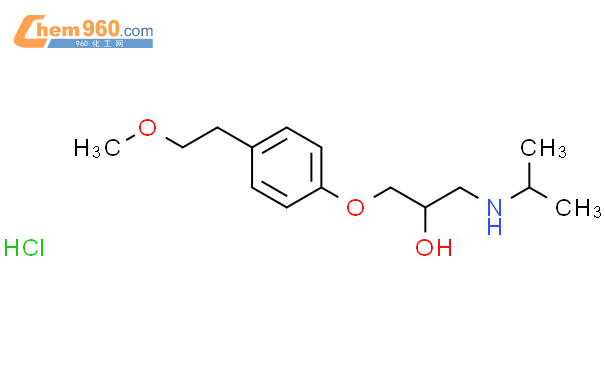
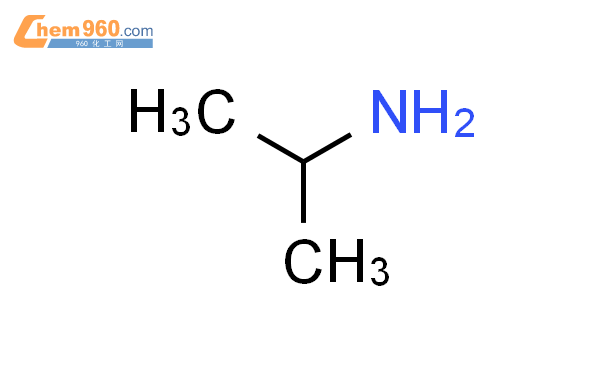
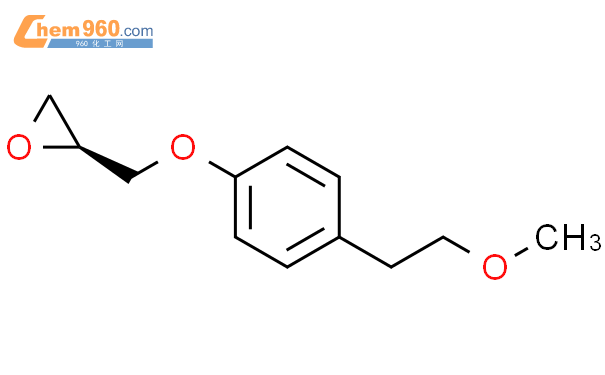
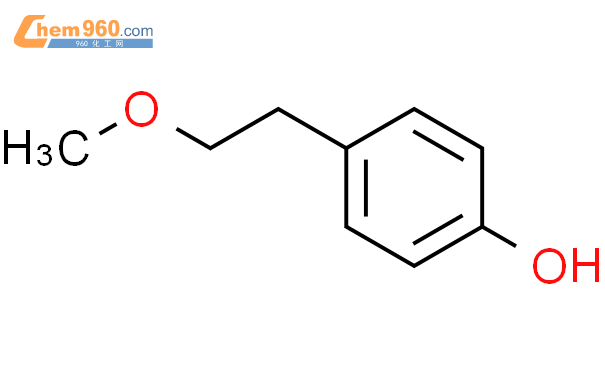
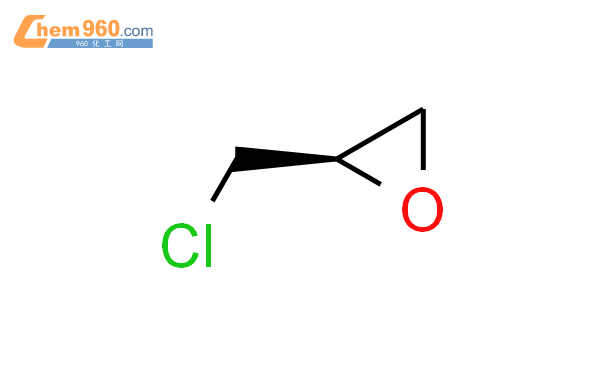
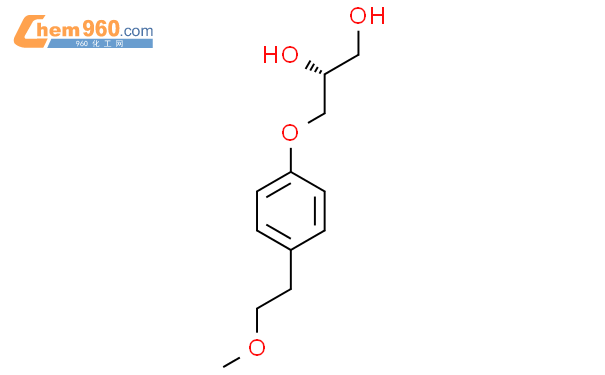
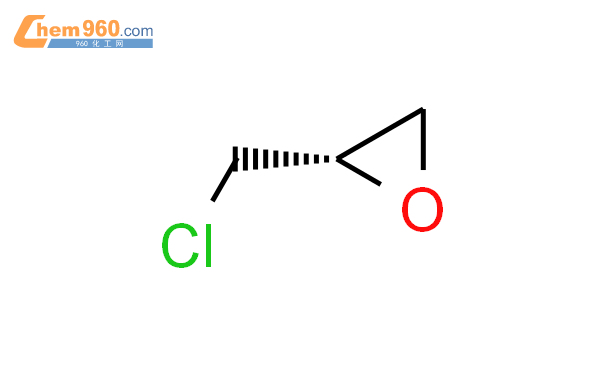
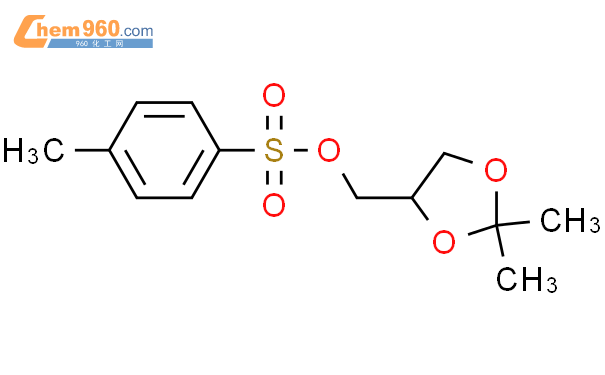
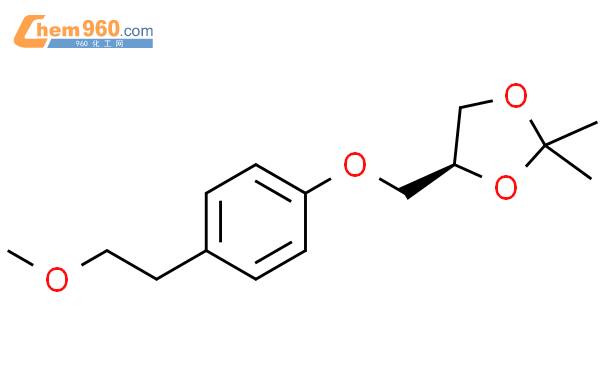
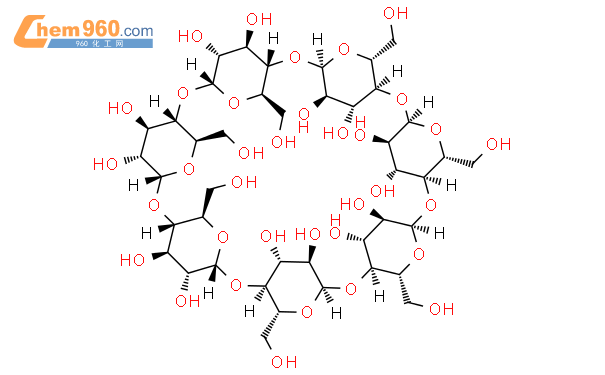
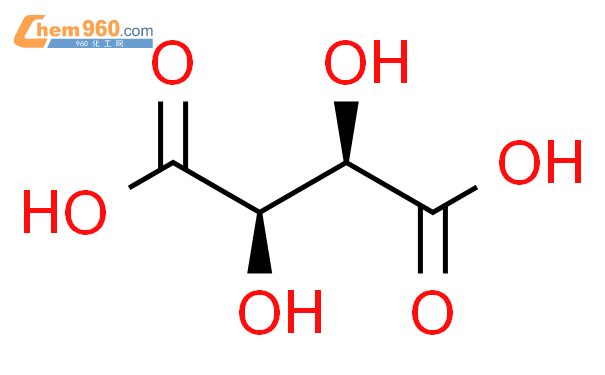
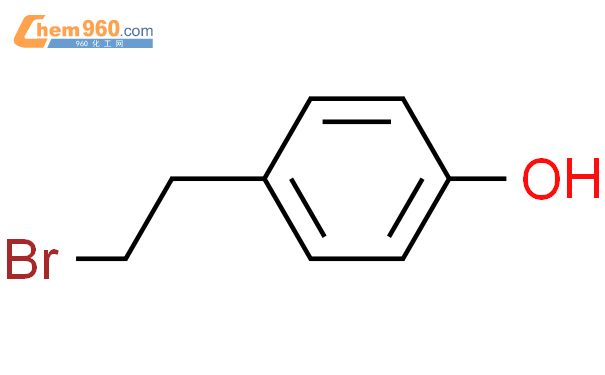
1.1
Reagents:
Hydrogen bromide
Solvents:
Water
2.1
Reagents:
Sodium methoxide
Solvents:
Methanol
3.1
Reagents:
Potassium hydroxide
Solvents:
Ethanol
4.1
Reagents:
Hydrochloric acid
Solvents:
Ethanol
5.1
Reagents:
Pyridine
Solvents:
Dichloromethane
6.1
Solvents:
Acetonitrile